Poster Presentation Australian Diabetes in Pregnancy Society 2018
Retrospective Audit of Pre-gestational and Gestational diabetes women receiving betamethasone at Bankstown-Lidcombe Hospital. (#114)
Introduction: Antenatal corticosteroids are used to accelerate lung maturity for several obstetric indications, including pre-term labour1. Betamethasone has the propensity to cause significant hyperglycemia, particularly in women with pre-gestational or gestational diabetes mellitus (GDM). This highlights the need for glycemic monitoring and involvement of the endocrine team to determine and institute appropriate glycaemic management strategies to prevent maternal hyperglycaemia.
Aim: We sought to evaluate current practice at Bankstown-Lidcombe Hospital with the aim of developing appropriate guidelines around betamethasone administration in pregnancy to ensure best practice.
Methods: We performed a retrospective audit of maternal records from May 2017 to May 2018 in consecutive women with pre-gestational diabetes or GDM receiving antenatal betamethasone. An equal number of pregnant women with normal glucose tolerance (also receiving betamethasone) acted as controls. Women requiring urgent betamethasone before an emergency caesarean section were excluded.
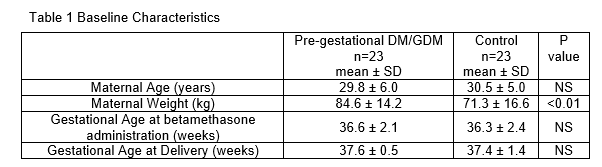
Results: There were 23 women in each group, with baseline characteristics shown in Table 1.
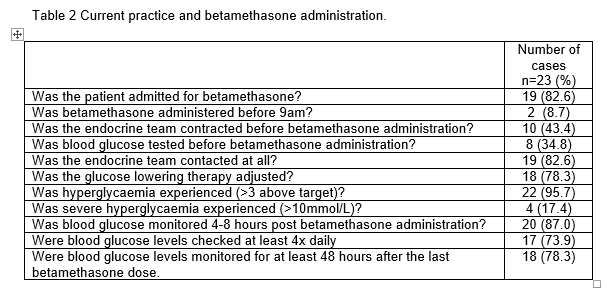
Identified areas for improvement were: timing of betamethasone administration, initiation of glucose monitoring and early involvement of the endocrine team prior to betamethasone administration. Significant hyperglycaemia was experienced in 22/23 pre-gestational DM/GDM women (95.7%), and severe hyperglycaemia (postprandial >10.0 mmol/L) in 4/23 women (17.4%). The median time between betamethasone administration and delivery was 2.8days (Range 0.0-67.2days). Table 2 includes other findings.
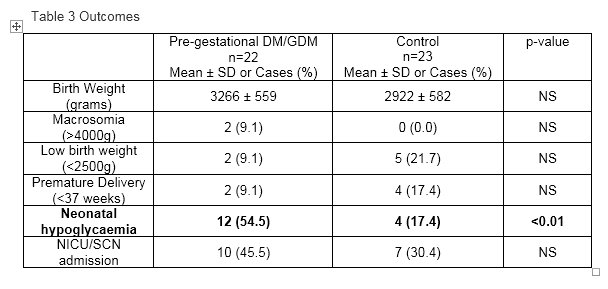
All but one Pre-gestational diabetes/GDM woman had delivered at the time of data collection. Neonatal hypoglycaemia was more common in Pre-gestational diabetes/GDM women, compared to NGT women 54.5% vs 17.4% (OR 5.7, 95% CI 1.5–22.2), despite no significant difference in birthweight or macrosomia rates. Results are summarised in Table 3.
Conclusion:
Our audit demonstrated that a structured guideline of glycaemic monitoring and management is required for pre-gestational DM and GDM women having betamethasone therapy. These women are at high risk of hyperglycaemia following betamethasone administration and their infants at high risk of neonatal hypoglycaemia.
- 1. Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, Saling E. Coordinators Of World Association of Perinatal Medicine Prematurity Working Group. Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med. 2008;36:191–6.